A Brief Explanation of Electrocoagulation
Coagulation is one of the most common, and most important, physico-chemical processes used in water treatment. As a basic description of these physico-chemical processes, all the reactions are interactions between electrons. In Electrocoagulation (EC), the water to be treated is flooded with electrons in which a number of additional reactions often occur during EC treatment, including (but not limited to):
- Ionization
- Hydrolysis
- Electrolysis
- Formation of free radicals
- Oxidation
- Reduction
- Disinfection
- Demulsification
- pH change
- Complexing/Bridging
Careful design of the EC reactor and control of the EC treatment process can somewhat enhance or minimize some of these reactions as necessary; however, some or all of these reactions may occur at some level during EC treatment. For this reason, EC is most economical when a large variety of contaminants need to be reduced by one unit process.
In Electrocoagulation, water is passed over metal electrodes and when electricity is applied to the electrodes, the metal starts reacting on an atomic level at the electrode-water interface to go from its neutral, elemental state to a charged state. This charged metal atom leaves the plate and enters the water. So, the electricity is applied to the electrodes to create the charged metal coagulant which bonds with dissolved contaminants, precipitates out of solution, organics adsorb to the precipitated solids, and solids agglomerate and separate. The electrode material is electrochemically consumed as treatment continues and must be periodically replaced.
Coagulation can be used to accomplish the following treatment goals:
- Dissolved organics can be removed by adsorption of the organic onto the precipitated solid matrix; level of removal depends on the type of organic, the constituents of the precipitant, and the concentrations of both.
- NH3 and PO4 removal
- Metals Precipitation: Coagulation can be used to remove many dissolved heavy metals (including iron, chromium, cadmium, nickel, zinc, copper, lead, and silver) by precipitating them out from the aqueous solution into a mostly non-soluble solid form.
BiO2 Solutions is utilizing BAKERCORP DBA KASELCO Electrocoagulation (EC) technology as a final polishing and redundant process to expand the type of wastewater applications in which we can be effective. Baker’s state-of-the-art EC technology includes multiple designs to enhance treatment, improve operations, and reduce treatment costs:
- Patented electrode cartridges improve speed and ease of removal and change-out.
- Proprietary power application methods and patented use of segmented plated means better treatment with less fouling with a smaller treatment system.
- Most efficient power consumption in the industry.
- Provide clean, treated water for discharge or reuse, reducing or eliminating the need for costly disposal and fresh water consumption.
- Self-cleaning feature without the use of chemicals.
EC technology can enhance your wastewater treatment system by:
- Economically treating many contaminants simultaneously, including microbiological contaminants.
- Producing water (and the resulting waste) which tends to be more stable and less reactive because reactions are pushed to their endpoints, which aids in reuse and/or disposal.
- For Lagoon Upset Control information, please click here to read about our Hydro, OK WWTP Installation.
EC technology requires solids separation and removal to be effective. Additionally, because the reactions occurring during EC treatment are so complex, treatment results should always be verified by bench testing.
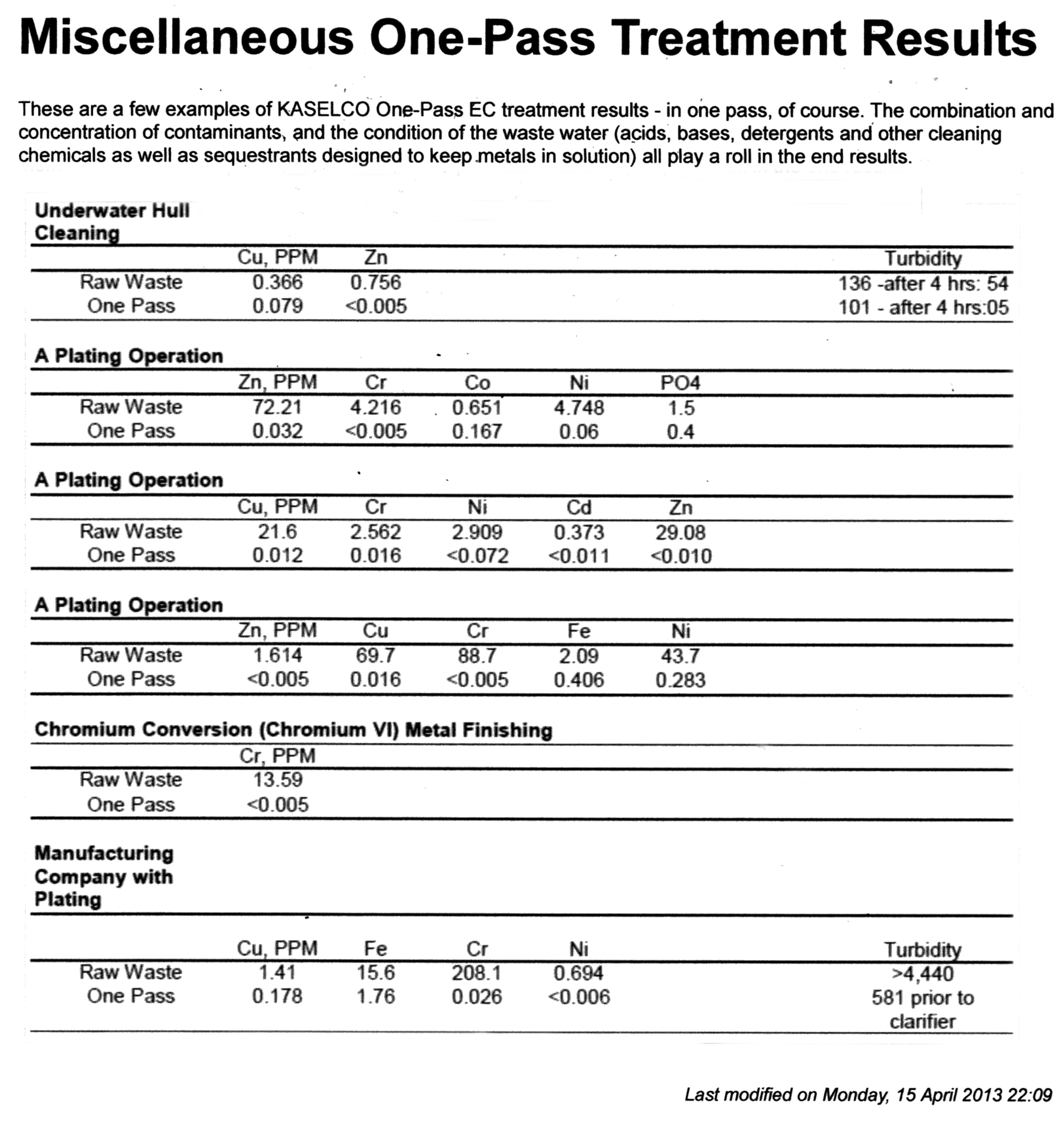
Industrial Water Treatment with Electrocoagulation
Plating wastewater is an excellent match for treatment with an electrocoagulation system. For example, a plating wastewater stream was treated with the EC treatment process. The water treated very well in the EC reactor and generated a good floc that settled well following treatment. A polymer was used to assist in floc formation in the sample. The metals concentrations in the treated water meet the discharge standards for the client. The metal concentrations in the raw and treated water are listed below.
| Sample | Zinc, ppm | Copper, ppm | Chromium, ppm | Iron, ppm | Nickel, ppm |
|---|---|---|---|---|---|
| Raw Water | 1.614 | 69.7 | 88.7 | 2.09 | 43.7 |
| Treated Water | <0.005 | 0.04 | <0.005 | <0.005 | 0.034 |
General Electrocoagulation Removal Rates